
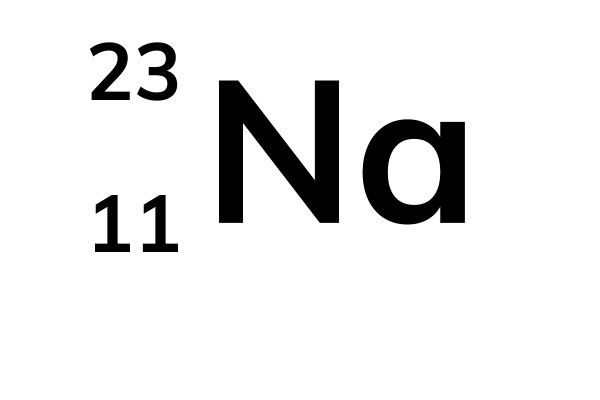
9 of them are naturally occurring, 4 of which are stable ones. In how many instances are the atomic weights of the elements out of order relative to the atomic numbers of. This element is part of solder, pewter, bearing metals, type metals and fusible alloys. Consider the stable elements through lead 1Z 822. It is used in building construction, lead-place accumulators, bullets and shot. Lead is a heavy dull grey ductile metallic element, belongs to group 14. There you can find the metals, semi-conductor (s), non-metal (s), inert noble gas (ses), Halogens, Lanthanoides. Please note that the elements do not show their natural relation towards each other as in the Periodic system. The unity for atomic mass is gram per mol. In such cases, chemists usually define a standard by arbitrarily assigning a numerical value to one of the quantities, which allows them to calculate numerical values for the rest.įigure 1.6.2 Determining Relative Atomic Masses Using a Mass Spectrometer. The lightest chemical element is Hydrogen and the heaviest is Hassium. We will encounter many other examples later in this text.

It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. Thus it is not possible to calculate absolute atomic masses accurately by simply adding together the masses of the electrons, the protons, and the neutrons, and absolute atomic masses cannot be measured, but relative masses can be measured very accurately. By measuring the relative deflection of ions that have the same charge, scientists can determine their relative masses (Figure 1.6.2). The extent of the deflection depends on the mass-to-charge ratio of the ion. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomson’s experiment. First, electrons are removed from or added to atoms or molecules, thus producing charged particles called ions. Lead is a chemical element of the periodic table with chemical symbol Pb and atomic number 82 with an atomic weight of 207.21 u and is classed as post-transition metal and is part of group 14 (carbon group).
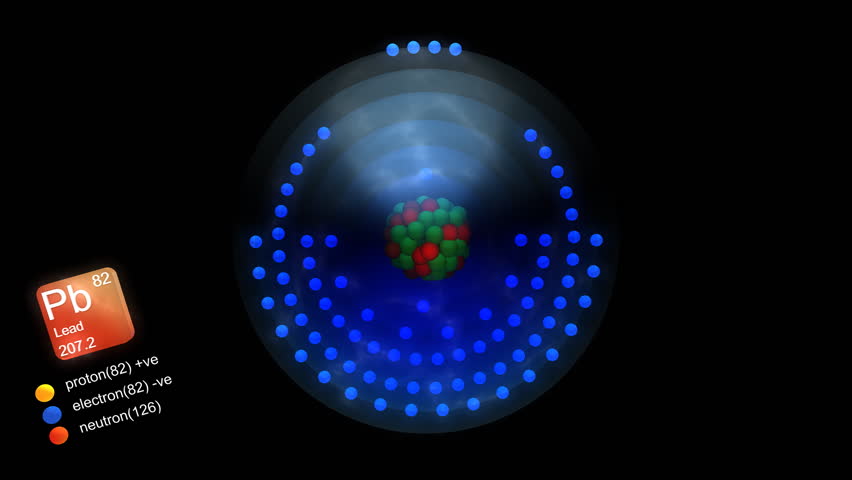
The technique is conceptually similar to the one Thomson used to determine the mass-to-charge ratio of the electron. Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. We can easily calculate the binding energy from the mass difference using Einstein's formula E=mc 2.īecause atoms are much too small to measure individually and do not have a charge, there is no convenient way to accurately measure absolute atomic masses. Although the difference in mass is small, it is extremely important because it is the binding energy of the nucleus. Lead is the p-block element and it belongs to carbon group.
#Lead atomic mass number free#
Free Vector Bookmark icon Collection Heart icon Favorite Share icon Share. Lead in Periodic table Lead element is in group 14 and period 6 of the Periodic table. Jump to main content Periodic Table Home History Alchemy Podcast Video Trends Periodic Table Home History Alchemy Podcast Video Trends You do not have JavaScript enabled. Chemical symbol with atomic number and atomic mass. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. Element Lead (Pb), Group 14, Atomic Number 82, p-block, Mass 207.2. Based on the average atomic mass of lead, which is 207, I think that Pb-207 is the most common isotope in lead, due to the similarity in mass. Br\) or, more commonly, 79Br and 81Br.Īlthough the masses of the electron, the proton, and the neutron are known to a high degree of precision (Table 1.5.1), the mass of any given atom is not simply the sum of the masses of its electrons, protons, and neutrons.


 0 kommentar(er)
0 kommentar(er)
